Abstract
Background Patients (pts) receiving ibrutinib (ibr) for CLL rarely achieve complete remission (CR) with undetectable minimal residual disease (U-MRD). Therefore, indefinite ibr maintenance therapy (Rx) is standard of care. Long-term Rx with ibr results in a cumulative risk of Rx discontinuation due to progression or toxicity. The risk of progression is highest in pts with complex karyotype and/or del(17p); some series suggest increased risk in pts with del(11q) or persistently elevated β2-microglobulin.
Venetoclax (ven) is synergistic with ibr. We hypothesized that adding ven to ibr in pts at high-risk for CLL progression, who had been on ibr for more than 12 months (mo) would achieve U-MRD and allow Rx discontinuation.
Methods We designed a phase II, investigator-initiated, response-adapted clinical trial with the addition of ven to ibr in pts (pts) with one or more high-risk features for disease progression who had received at least 1y of ibr regardless of line of Rx. Pts had detectable disease at time of study entry without meeting IWCLL criteria for progression. High-risk was defined as presence of at least one of: del(17p) or TP53 mutation; complex karyotype; del(11q); or elevated β2-microglobulin. Ibr was continued at 140-420mg/d and standard initiation and escalation of ven was performed to the target dose of 400mg/d. Rx with combined ibr and ven continued for a maximum duration of 24, 28-day cycles (C). Pts had bone marrow (BM) evaluation for MRD by flow cytometry (sensitivity 10-4) and CT scan for re-staging every 6mo. Pts in CR with U-MRD on two consecutive evaluations, 6mo apart, stopped ven, but could continue ibr at physician discretion. Pts who were MRD+ post-C24 continued ibr maintenance.
Results: We report the results of the first 45 pts enrolled. All patients have completed ven treatment. Median number of prior therapies was 1 (range 0-4). 22/45 patients were receiving ibr as initial Rx. 63% of pts had del(17p) and/or mutated TP53.
On intent to treat analysis, 17/45 pts (38%) post-C6 and 26/45 (57%) post-C12 achieved U-MRD. Three patients stopped ven prior to C12 (ineligible, n=1, metastatic solid tumor, n=2). Six of 16 (38%) patients with MRD+ at C12, converted to U-MRD by the end of the study. Best cumulative rate of U-MRD in BM was 33/45 (73%), Figure 1A. There was no difference in U-MRD4 rates or PFS according in those with TP53 abnormalities vs those without. Three pts were in CR prior to ven. Of the remaining pts, 24/42 (57%) pts improved response to CR/CRi during ven treatment.
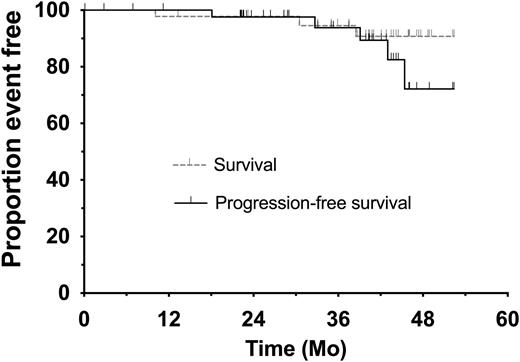
Median follow-up is 37.5 mo for PFS and 40.3 mo for OS. Forty-one patients completed treatment per protocol. Of the 32 patients with U-MRD at the completion of Rx, 10 stopped ven prior to C24 per protocol (5 after C12 and 5 after C18) after achieving U-MRD CR on two consecutive response assessments. At completion of ven, 10/32 pts with U-MRD4 continued ibr at physician discretion (6/10 with del(17p)). Five patients have progressed (Figure 1B): 1 during combination therapy; 3 during ibr maintenance (2 of whom had U-MRD4 at completion of ven); 1 with Richter's Syndrome during observation after achieving U-MRD4 CR. No pt died during ven Rx. Two patients died after receiving treatment for other cancers (metastatic melanoma, AML) and one after coming off protocol to receive acalabrutinib.
Blood MRD analysis was obtained q6 mo (Figure 1C) in pts with U-MRD at the EOT; 10/32 have had confirmed MRD re-emergence (8/22 who stopped ibrutinib and 2/10 who continued); 3 subsequently progressed.
Treatment was well-tolerated. The most common AE was diarrhea in 47 pts, grade 1-2 in all but 1 pt. The most common grade 3-4 AE was neutropenia, seen in 20% pts (Grade 3 in 8 and grade 4 in 1 patient). No febrile neutropenia was seen. Three patients developed grade 3 infections (pneumonia, pyelonephritis, skin abscess). No patient stopped ven due to toxicity. Seventeen patients experienced at least one SAE, most commonly non-melanoma skin cancers (n=9).
Conclusions: Consolidation ven added to ibr in pts with high-risk CLL was well-tolerated and achieved cumulative BM U-MRD4 rate of 73% ibr discontinuation in 49% of pts. Only 1/45 pts progressed during combination Rx. At a median of 12 mo post-ven follow up, most pts who attained BM U-MRD have ongoing U-MRD in blood. A second cohort restricted to 45 high-risk pts with TP53 abnormalities or complex karyotype is accruing and there are plans to also include patients treated with acalabrutinib.
Disclosures
Thompson:AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech: Consultancy; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech, Amgen: Honoraria; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AbbVie, Pharmacyclics, Adaptive Biotechnologies, Genentech: Research Funding. Ferrajoli:Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Research Funding. Jain:Servier Pharmaceuticals LLC: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; ADC Therapeutics: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Cellectis: Honoraria, Research Funding; Incyte Corporation: Research Funding; Pfizer: Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Mingsight: Research Funding; Takeda: Research Funding; Medisix: Research Funding; Loxo Oncology: Research Funding; Novalgen: Research Funding; Dialectic Therapeutics: Research Funding; Newave: Research Funding; TransThera Sciences: Research Funding; Beigene: Honoraria; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; MEI Pharma: Honoraria; Ipsen: Honoraria; CareDx: Honoraria. Kadia:Glycomimetics: Research Funding; Iterion: Research Funding; PinotBio: Consultancy; Delta-Fly: Research Funding; cyclacel: Research Funding; Amgen: Research Funding; AstraZeneca: Research Funding; Astellas: Research Funding; Genfleet: Research Funding; Ascentage: Research Funding; cellenkos: Research Funding; Servier: Consultancy; Pfizer: Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding; Regeneron: Research Funding; Astex: Honoraria. Bose:Novartis: Honoraria; CTI BioPharma: Honoraria, Research Funding; NS Pharma: Research Funding; Kartos: Research Funding; Pfizer: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; Astellas: Research Funding; Ionis: Research Funding; Telios: Research Funding; Promedior: Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Disc Medicine: Research Funding; Cogent: Honoraria, Research Funding; Pharma Essentia: Honoraria; Sierra Oncology (now GSK): Consultancy; AbbVie: Consultancy; Karyopharm: Consultancy; BMS: Consultancy, Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Short:AstraZeneca: Consultancy; Novartis: Consultancy; Amgen: Consultancy, Honoraria; Takeda Oncology: Consultancy, Research Funding; Stemline Therapeutics: Research Funding; Pfizer: Consultancy; Astellas: Research Funding. Wierda:Karyopharm: Research Funding; Juno: Research Funding; Genzyme: Consultancy; Sanofi: Consultancy; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Kite, a Gilead Company: Research Funding; Xencor: Research Funding; Sunesis: Research Funding; Pharmacyclics LLC: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Janssen: Research Funding; GSK/Novartis: Research Funding; Gilead Sciences: Research Funding; Genentech: Research Funding; Cyclacel: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; AbbVie: Research Funding.
OffLabel Disclosure:
Venetoclax added to ibrutinib in CLL
Author notes
Asterisk with author names denotes non-ASH members.